Australia is home to a unique bunch of native land mammals, such as koalas, wombats and wallabies. These furballs evolved in isolation on this island continent and have become Australian symbols.
But between 27 and 23 million years ago, the coastal seas of Australia were also home to sea mammals found almost nowhere else: whales.
But not just any old whales. These creatures were among the strangest of all whales, called mammalodontids. If alive today, mammalodontids would be as iconically Australian as kangaroos.
Recent fossil discoveries from coastal Victoria reveal that not just one or two species, but a cornucopia of these wonderfully weird whales once called Australia home.
Our latest find, a roughly 25-million-year-old fossil of a newly named whale species Janjucetus dullardi, joins their bizarre ranks. Our discovery is published today in the Zoological Journal of the Linnean Society.
Baleen whales without baleen
Today, some of the most iconic whale species, such as blue and humpback whales, are baleen whales. These ocean giants use hair-like structures in their mouths, called baleen, to filter plankton – their main food source.
By contrast, mammalodontids were small-bodied (no longer than three metres), big-eyed, and had short jaws lined with teeth. Despite this description, we know that mammalodontids were, in fact, baleen whales … that lacked baleen. They were like an offshoot from the main evolutionary branch leading to today’s toothless giants.
All mammalodontid fossils date from the late Oligocene epoch – 27 to 23 million years ago. And three out of four named species have been found on Victoria’s Surf Coast, south-east of Melbourne.
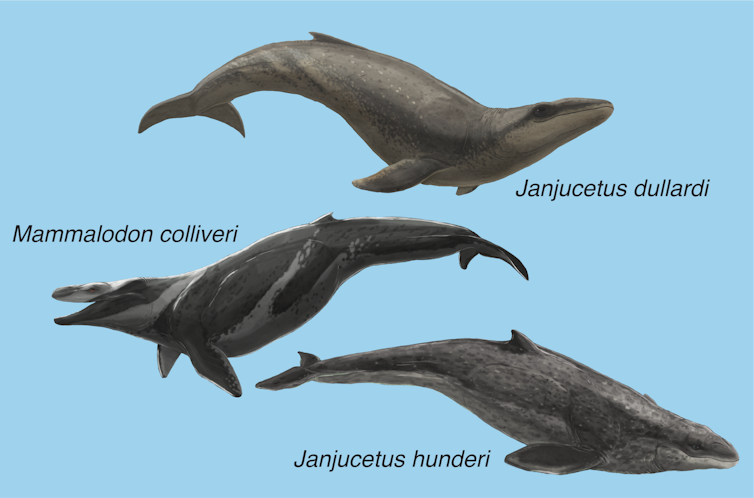
The first mammalodontid was found in 1932, and in 1939 was given the name Mammalodon colliveri. It had blunt jaw bones with extensive blood and nerve supply for face and lip muscles. Curiously, the teeth were worn down to the gums, suggesting it fed by slurping prey (along with abrasive grit) from the seabed.
In 2006, local naturalist Staumn Hunder found the first fossil of a species later named after him, Janjucetus hunderi. This whale sported a robust triangular snout with sharp teeth and powerful jaw-closing muscles.
If Mammalodon’s skull proclaims “I’m a sucker”, the skull of Janjucetus screams “biter and ripper”. It’s about as heavy metal as whale skulls get, departing radically from those of all other whales.
Although Mammalodon colliveri and Janjucetus hunderi hint at a surprisingly wide range of lifestyles for mammalodontids, the details of exactly how and when they became so different from other whales remain murky.

A tiny new whale
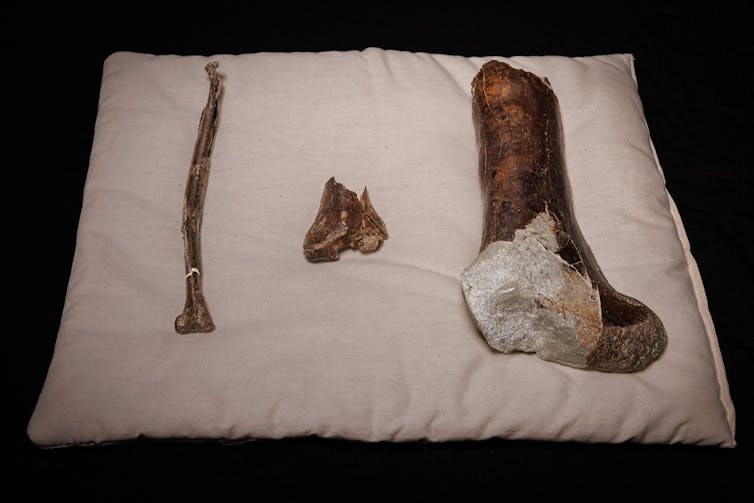
In 2019, school principal Ross Dullard found a whale fossil eroding out of rocks along the coast at Jan Juc in Victoria.
Dullard donated his find to Museums Victoria, where it was painstakingly cleaned and repaired in the laboratory so we could study it.
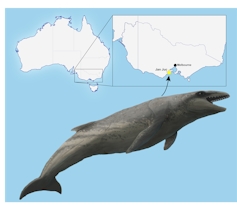
As we describe in our new paper, Dullard’s find is a mammalodontid like Janjucetus hunderi, yet with different enough teeth and ear bones to warrant the naming of a new species: Janjucetus dullardi.
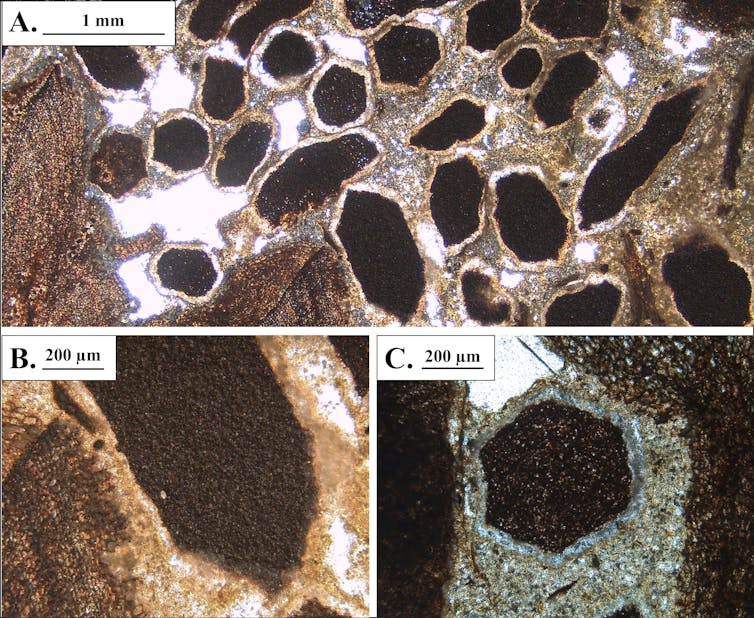
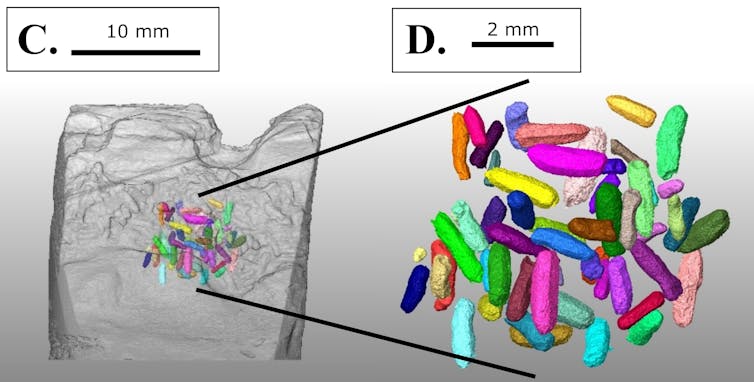
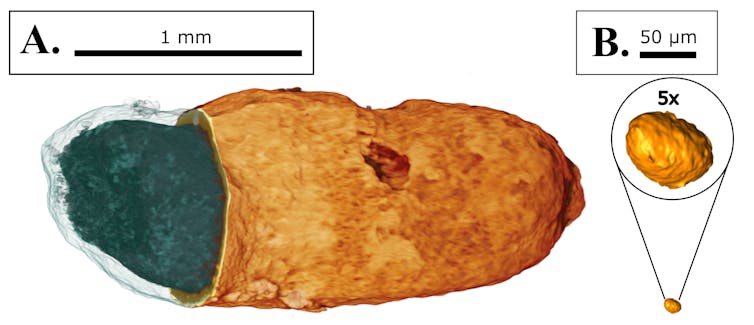
Incomplete fusion between skull bones, minimal tooth wear, and open tooth root canals tell us the animal was not fully grown when it died, possibly being a juvenile.
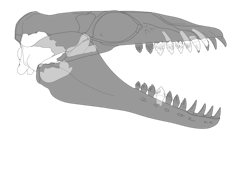
But just how small was it?
Using an equation that takes into account measurements of skull width compared with the total length of whales, we predicted that Janjucetus dullardi was about two metres long – small enough to fit on a standard single bed.
This makes it the smallest fossil whale discovered in Australia, and perhaps the first fossil of a juvenile whale found here.

A warm-water paradise
Janjucetus dullardi and its fellow mammalodontids lived during the Late Oligocene Warming, between 26 and 23 million years ago. The coastal waters of Victoria were as warm as those off present-day northeast New South Wales, and the sea level was higher.
Small, toothy whales clearly didn’t mind this long summer of balmy, sunlit waters: 80% of the dozens of whale fossils found in Victoria from that era are mammalodontids – mostly unnamed species. In contrast, rocks of the same age in New Zealand have yielded just one mammalodontid from a century of intensive fossil whale collecting.
Unfortunately, the mammalodontid paradise was lost. By about 22 million years ago, mammalodontids had gone extinct, no longer playing a part in the ongoing saga of baleen whale evolution. Global cooling at about 23 million years ago resulted in lower sea levels and the loss of the mammalodontids’ shallow coastal habitat.

If we know how their story ends, the beginning is still a mystery. Our research on Janjucetus dullardi and its kin suggests mammalodontids must have originated long before the age of their oldest known fossils, maybe 34 million years ago.
We suspect that the cradle of their evolution was here, in splendid isolation off southern Australia – home of the mammalodontids.![]()
Erich Fitzgerald, Senior Curator, Vertebrate Palaeontology, Museums Victoria Research Institute and Ruairidh Duncan, PhD Candidate, Palaeontology, Monash University
This article is republished from The Conversation under a Creative Commons license. Read the original article.